CDC Director Rochelle P. Walensky, M.D., M.P.H., endorsed the CDC Advisory Committee on Immunization Practices’ (ACIP) recommendations for use of updated COVID-19 boosters from Pfizer-BioNTech for people ages 12 years and older and from Moderna for people ages 18 years and older.
Updated COVID-19 boosters add Omicron BA.4 and BA.5 spike protein components to the current vaccine composition, helping to restore protection that has waned since previous vaccination by targeting variants that are more transmissible and immune-evading.
In the coming weeks, CDC also expects to recommend updated COVID-19 boosters for other pediatric groups, per the discussion and evaluation of the data by ACIP on Sept. 1, 2022. When data are available and FDA authorizes these other types of COVID-19 boosters, CDC will quickly move to help make them available in the United States.
The Food and Drug Administration’s (FDA) authorization of updated COVID-19 boosters, and CDC’s recommendation for use, are critical next steps forward in our country’s vaccination program—a program that has helped provide increased protection against COVID-19 disease and death.
Convalescent plasma generated great enthusiasm in the earliest days of the coronavirus disease 2019 (covid-19) pandemic because of a plausible mechanism of action,1 its 100 year history of use in the treatment of other infectious diseases,2 and rapid availability from voluntary donors.3
In the linked PLACID Trial, Agarwal and colleagues (doi:10.1136/bmj.m3939) evaluated convalescent plasma for the treatment of moderate covid-19 in patients admitted to hospital in India.4 Strengths of the study included a primary “hard” outcome meaningful to patients, “real world” patient enrollment with no exclusions for comorbidities, careful attention to donor selection and safety screening of donated plasma, post facto quantitative testing of antibody titers in all plasma samples, assessment of secondary patient outcomes, and evaluation of the efficacy of the subsample of plasma donations that contained detectable titers of antibodies to severe acute respiratory coronavirus 2 (SARS-CoV-2), the virus responsible for covid-19.
In prespecified, intention-to-treat analyses, the PLACID Trial investigators found no net benefit associated with convalescent plasma in patients admitted to hospital with moderate covid-19. The composite primary outcome (progression to severe disease or all cause mortality at 28 days) occurred in 19% (44/235) of patients in the intervention arm and 18% (41/229) of patients in the control arm (risk ratio 1.04, 95% confidence interval 0.71 to 1.54). Restricting the comparison to the subset of patients who received plasma with detectable antibody titers did not change the outcome.4
Small beneficial effects were found for resolution of shortness of breath and fatigue. However, these results should be interpreted with caution, because the trial was not blinded, so knowledge of treatment status could have influenced the reporting of subjective symptoms by patients who survived to day 7.
The primary hypothesized mechanism of benefit from convalescent plasma is through direct antiviral action of neutralizing antibodies on SARS-CoV-2 RNA.1 In the PLACID Trial, a statistically significant 20% higher rate of conversion to a negative result for SARS-CoV-2 RNA occurred on day 7 among patients in the intervention arm.
In plain English, this means that convalescent plasma did exactly what the investigators hoped it would do, yet there was no net clinical benefit to patients. Why might this be the case?
The most common use of therapeutic plasma, which contains more than 1000 different proteins,1 is for the management of acute bleeding and complex coagulopathies.5 Despite the presence in plasma of anticoagulation factors such as antithrombin and protein C, the net effect of plasma is prothrombotic.5 Immunoglobulin therapy, which is derived from whole plasma, is subject to a US Food and Drug Administration warning about the risks of thrombosis, particularly in older patients, those with cardiovascular risk factors, and those with hypercoagulable conditions.5
It is now widely recognized that covid-19 is a life threatening thrombotic disorder.678 An excellent recent pathophysiology synthesis concluded that “SARS-CoV-2 not only produces an inflammatory and hypercoagulable state, but also a hypofibrinolytic state not seen with most other types of coagulopathy.”7 Most recently, plasma from convalescent covid-19 patients has been shown to directly cause endothelial cell damage in vitro.9
Following suggestions of benefit from observational studies, convalescent plasma was given to more than 100 000 patients admitted to hospital with covid-19 in the US between April and August—under the FDA’s expanded access treatment protocol.10 The authors of the safety update on the first 20 000 recipients said their results provided “robust evidence that transfusion of CP [convalescent plasma] is safe.”11
However, close examination reveals that adjudication of the “relatedness” of serious adverse thrombotic and cardiac events was conducted by the treating physician, with no defined protocol and no independent review.11 Most of the 677 cardiac events (88.2%) and 113 thrombotic events (66.3%) were judged not to be related to transfusion, and these events were therefore excluded from the reported adverse event rates.
Thrombotic events were not a prespecified outcome in the PLACID Trial and were not reported. Nonetheless, it is notable that progression to severe disease or death occurred in 20% (13/64) of patients who received convalescent plasma with no detected neutralizing antibodies compared with 18% (41/229) of controls.
The PLACID Trial was a rigorous randomized controlled study on a topic of enormous global importance, ethically designed and implemented given the contemporaneous state of scientific knowledge about SARS-CoV-2.4 With publication of the findings, the bar has been raised for all ongoing and future trials.
The following recommendations should be carefully considered by both safety monitoring and institutional review boards in light of the PLACID Trial findings: First, the potential harms of the non-immune components of convalescent plasma should be rigorously investigated, especially prothrombotic risks, and considered when choosing trial outcomes and participant exclusion criteria. Second, only donor plasma with detectable titers of neutralizing antibodies should be given to trial participants, to ensure that the potential for benefit exists for all intervention arm patients. Third, double blind designs with sham procedure controls should be the gold standard for future trials. Low risk sham procedures can be ethically acceptable under prescribed conditions.12
Fourth, non-immune plasma should not be used as a control intervention, because of potential harms and availability of lower risk alternatives such as normal saline.
The desperation engendered by covid-19 demands that we strongly resist the urge to succumb to pandemic research exceptionalism.13 High quality clinical research must be an integral part of a coordinated international response.13 Specifically, scientific validity is a necessary component of ethical research.14 Low quality research not only wastes scarce resources, it is also inherently unethical.14
Finally, when multiple research teams require participants, triage committees are needed to direct recruitment away from low priority, duplicative, or underpowered trials with little potential for usable findings.1315 Institutions must guarantee that patients with covid-19 are informed of all available trial options and assured autonomy in their decisions about participation.15
Source: British Medical Journal | Medical Editorial | Convalescent Plasma Is Ineffective For COVID-19
Research Abstract
Objective:
To investigate the effectiveness of using convalescent plasma to treat moderate coronavirus disease 2019 (covid-19) in adults in India.
Design:
Open label, parallel arm, phase II, multicentre, randomised controlled trial.
Setting:
39 public and private hospitals across India.
Participants:
464 adults (≥18 years) admitted to hospital (screened 22 April to 14 July 2020) with confirmed moderate covid-19 (partial pressure of oxygen in arterial blood/fraction of inspired oxygen (PaO2/FiO2) ratio between 200 mm Hg and 300 mm Hg or a respiratory rate of more than 24/min with oxygen saturation 93% or less on room air): 235 were assigned to convalescent plasma with best standard of care (intervention arm) and 229 to best standard of care only (control arm).
Interventions:
Participants in the intervention arm received two doses of 200 mL convalescent plasma, transfused 24 hours apart. The presence and levels of neutralising antibodies were not measured a priori; stored samples were assayed at the end of the study.
Main outcome measure:
Composite of progression to severe disease (PaO2/FiO2 <100 mm Hg) or all cause mortality at 28 days post-enrolment.
Results:
Progression to severe disease or all cause mortality at 28 days after enrolment occurred in 44 (19%) participants in the intervention arm and 41 (18%) in the control arm (risk difference 0.008 (95% confidence interval −0.062 to 0.078); risk ratio 1.04, 95% confidence interval 0.71 to 1.54).
Conclusion:
Convalescent plasma was not associated with a reduction in progression to severe covid-19 or all cause mortality. This trial has high generalisability and approximates convalescent plasma use in real life settings with limited laboratory capacity. A priori measurement of neutralising antibody titres in donors and participants might further clarify the role of convalescent plasma in the management of covid-19.
Trial registration:
Clinical Trial Registry of India CTRI/2020/04/024775.
Read the full source medical article here: British Medical Journal | Research Paper | Convalescent plasma in the management of moderate covid-19 in adults in Indiea: open label phase II multicentre randomised controlled trial (PLACID Trial).
The largest trial to date of treatments repurposed for use in the covid-19 pandemic has shown that none of the four drugs studied produced any measurable benefit in mortality or disease course. This includes remdesivir—a drug already recommended by several guidelines and pre-ordered by numerous governments around the world.
Hydroxychloroquine, lopinavir-ritonavir, and interferon beta-1a regimens also seemed to have little or no effect on 28 day mortality. None of the drugs delayed the need for ventilation or shortened the stay of patients admitted to hospital. “For each drug in the study, the effect on mortality was disappointingly unpromising,” said the World Health Organization in a statement.
The WHO Solidarity trial followed 11 266 adults at 405 hospitals in 30 countries and, although the results are preliminary, WHO said that the “conclusive” findings “suffice to refute early hopes” in the four drugs studied. The study, which awaits peer review before publication in a medical journal, has been posted on the preprint website medrxiv.org.1
None of the drugs showed any real trend towards improved survival, even a non-significant one. The closest approach to statistical significance was a non-significant trend towards lower survival in patients who took hydroxychloroquine or interferon beta-1a.
The largest previous trial of remdesivir in covid-19, the ACTT-1 study sponsored by the US National Institutes of Health, found a significant benefit in time to recovery and what remdesivir’s maker, Gilead, called a non-significant trend towards improved survival at 29 days (hazard ratio 0.73 (95% confidence interval 0.52 to 1.03)). That trial, which published its final report last week,2 led to the drug’s authorisation in the UK, the EU, and the US.
The WHO trial followed five times as many patients taking remdesivir and 10 times as many overall. “The trial is beautiful in its simplicity and clarity of purpose,” said Martin Landray, an Oxford University epidemiologist. He said that the poor results seen with hydroxychloroquine and lopinavir-ritonavir were expected, as they had previously failed to show a benefit in the UK Recovery trial, which Landray leads.
“The result for interferon is interesting, and there will doubtless be some debate about whether different doses or routes of administration—for example, by nebuliser—might be more effective,” he said. “But the big story is the finding that remdesivir produces no meaningful impact on survival.”
He explained that “people will argue about the need for earlier use” of remdesivir but that, even if it brought modest benefits, “the absolute numbers of lives saved would be small.”
Landray added, “Remember too that remdesivir is a drug that is given by intravenous infusion for five to 10 days and costs around £2000 [€2205; $2600] per course. Covid affects millions of people. It is not a rare disease. We need scalable, affordable, and equitable treatments. The WHO Solidarity trial has done the world a huge favour by producing clear, independent, and robust results.”
Gilead said in a statement that it was “concerned that the data from this open-label global trial have not undergone the rigorous review required to allow for constructive scientific discussion.”
WHO said that the Solidarity trial, which continues to recruit about 2000 patients a month, may expand to investigate newer antiviral drugs, immunomodulators, and anti-SARS-CoV-2 monoclonal antibodies.
Hydroxychloroquine and chloroquine have been proposed as treatments for coronavirus disease 2019 (Covid-19) on the basis of in vitro activity and data from uncontrolled studies and small, randomized trials.
In this randomized, controlled, open-label platform trial comparing a range of possible treatments with usual care in patients hospitalized with Covid-19, we randomly assigned 1561 patients to receive hydroxychloroquine and 3155 to receive usual care. The primary outcome was 28-day mortality.
The enrollment of patients in the hydroxychloroquine group was closed on June 5, 2020, after an interim analysis determined that there was a lack of efficacy. Death within 28 days occurred in 421 patients (27.0%) in the hydroxychloroquine group and in 790 (25.0%) in the usual-care group (rate ratio, 1.09; 95% confidence interval [CI], 0.97 to 1.23; P=0.15). Consistent results were seen in all prespecified subgroups of patients. The results suggest that patients in the hydroxychloroquine group were less likely to be discharged from the hospital alive within 28 days than those in the usual-care group (59.6% vs. 62.9%; rate ratio, 0.90; 95% CI, 0.83 to 0.98). Among the patients who were not undergoing mechanical ventilation at baseline, those in the hydroxychloroquine group had a higher frequency of invasive mechanical ventilation or death (30.7% vs. 26.9%; risk ratio, 1.14; 95% CI, 1.03 to 1.27). There was a small numerical excess of cardiac deaths (0.4 percentage points) but no difference in the incidence of new major cardiac arrhythmia among the patients who received hydroxychloroquine.
Among patients hospitalized with Covid-19, those who received hydroxychloroquine did not have a lower incidence of death at 28 days than those who received usual care. (Funded by UK Research and Innovation and National Institute for Health Research and others; RECOVERY ISRCTN number, ISRCTN50189673. opens in new tab; ClinicalTrials.gov number, NCT04381936. opens in new tab.)
Read the full source study here: The New England Journal of Medicine | Medical Article | Effect of Hydroxychloroquine in Patients with COVID-19
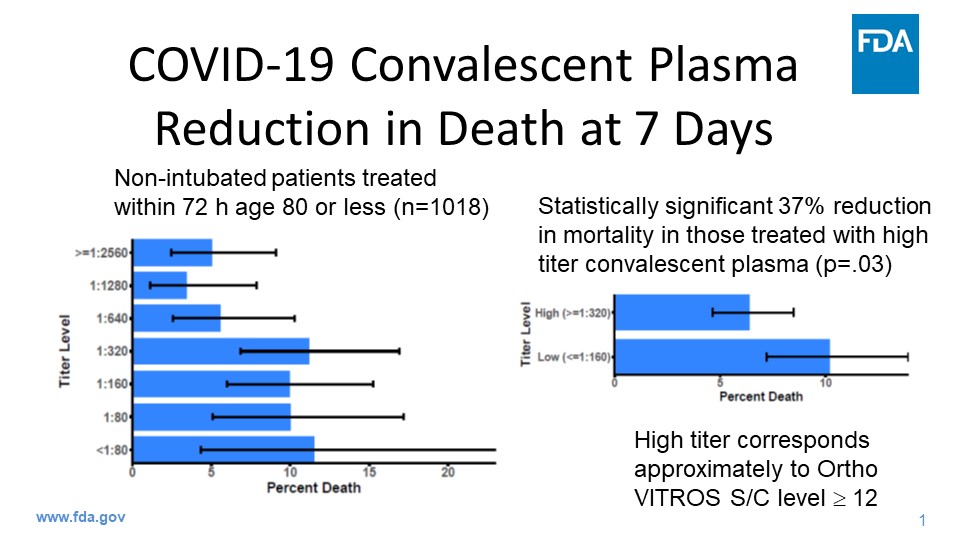
Today, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for investigational convalescent plasma for the treatment of COVID-19 in hospitalized patients as part of the agency’s ongoing efforts to fight COVID-19. Based on scientific evidence available, the FDA concluded, as outlined in its decision memorandum, this product may be effective in treating COVID-19 and that the known and potential benefits of the product outweigh the known and potential risks of the product.
Today’s action follows the FDA’s extensive review of the science and data generated over the past several months stemming from efforts to facilitate emergency access to convalescent plasma for patients as clinical trials to definitively demonstrate safety and efficacy remain ongoing.
The EUA authorizes the distribution of COVID-19 convalescent plasma in the U.S. and its administration by health care providers, as appropriate, to treat suspected or laboratory-confirmed COVID-19 in hospitalized patients with COVID-19.
Alex Azar, Health and Human Services Secretary:
“The FDA’s emergency authorization for convalescent plasma is a milestone achievement in President Trump’s efforts to save lives from COVID-19,” said Secretary Azar. “The Trump Administration recognized the potential of convalescent plasma early on. Months ago, the FDA, BARDA, and private partners began work on making this product available across the country while continuing to evaluate data through clinical trials. Our work on convalescent plasma has delivered broader access to the product than is available in any other country and reached more than 70,000 American patients so far. We are deeply grateful to Americans who have already donated and encourage individuals who have recovered from COVID-19 to consider donating convalescent plasma.”
Stephen M. Hahn, M.D., FDA Commissioner:
“I am committed to releasing safe and potentially helpful treatments for COVID-19 as quickly as possible in order to save lives. We’re encouraged by the early promising data that we’ve seen about convalescent plasma. The data from studies conducted this year shows that plasma from patients who’ve recovered from COVID-19 has the potential to help treat those who are suffering from the effects of getting this terrible virus,” said Dr. Hahn. “At the same time, we will continue to work with researchers to continue randomized clinical trials to study the safety and effectiveness of convalescent plasma in treating patients infected with the novel coronavirus.”
Scientific Evidence on Convalescent Plasma
Based on an evaluation of the EUA criteria and the totality of the available scientific evidence, the FDA’s Center for Biologics Evaluation and Research determined that the statutory criteria for issuing an EUA criteria were met.
The FDA determined that it is reasonable to believe that COVID-19 convalescent plasma may be effective in lessening the severity or shortening the length of COVID-19 illness in some hospitalized patients. The agency also determined that the known and potential benefits of the product, when used to treat COVID-19, outweigh the known and potential risks of the product and that that there are no adequate, approved, and available alternative treatments.
The EUA is not intended to replace randomized clinical trials and facilitating the enrollment of patients into any of the ongoing randomized clinical trials is critically important for the definitive demonstration of safety and efficacy of COVID-19 convalescent plasma. The FDA continues to recommend that the designs of ongoing randomized clinical trials of COVID-19 convalescent plasma and other therapeutic agents remain unaltered, as COVID-19 convalescent plasma does not yet represent a new standard of care based on the current available evidence.
Terms of EUA
The EUA requires that fact sheets providing important information about using COVID-19 convalescent plasma in treating COVID-19 be made available to health care providers and patients, including dosing instructions and potential side effects. Possible side effects of COVID-19 convalescent plasma include allergic reactions, transfusion-associated circulatory overload, and transfusion associated lung injury, as well as the potential for transfusion-transmitted infections.
Mayo Clinic Expanded Access Program
The FDA initially facilitated access to convalescent plasma for treating COVID-19 by using pathways that included traditional clinical trials and emergency single-patient investigational new drug (IND) applications.
An Expanded Access ProgramExternal Link Disclaimer for convalescent plasma was initiated in early April to fill an urgent need to provide patient access to a medical product of possible benefit during a time that the FDA was working with researchers to facilitate the initiation of randomized clinical trials to study convalescent plasma. As the number of single patient IND requests started to number in the hundreds on a daily basis, the FDA worked collaboratively with industry, academic, and government partners to implement an expanded access protocol to provide convalescent plasma to patients in need across the country via the national expanded access treatment protocol. The program was developed with funding from the HHS’ Biomedical Advanced Research and Development Authority (BARDA), with the Mayo Clinic serving as the lead institution. To date, the program has facilitated the infusion of over 70,000 patients with convalescent plasma.
The EUA was issued to the HHS Office of the Assistant Secretary for Preparedness and Response.
The EUA remains in effect until the termination of the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biologics for prevention and treatment of COVID-19. The EUA may be revised or revoked if it is determined the EUA no longer meets the statutory criteria for issuance.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
Study Name:
Association of Race With Mortality Among Patients Hospitalized With Coronavirus Disease 2019 (COVID-19) at 92 US Hospitals
Question:
Is race associated with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) in the United States?
Findings:
In this cohort study of 11 210 individuals with COVID-19 presenting for care at 92 hospitals across 12 states, there was no difference in all-cause, in-hospital mortality between White and Black patients after adjusting for age, sex, insurance status, comorbidity, neighborhood deprivation, and site of care.
Meaning:
In this study, race was not independently associated with in-hospital mortality after adjusting for differences in sociodemographic and clinical factors.
Today, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) to Yale School of Public Health for its SalivaDirect COVID-19 diagnostic test, which uses a new method of processing saliva samples when testing for COVID-19 infection.
“The SalivaDirect test for rapid detection of SARS-CoV-2 is yet another testing innovation game changer that will reduce the demand for scarce testing resources,” said Assistant Secretary for Health and COVID-19 Testing Coordinator Admiral Brett P. Giroir, M.D. “Our current national expansion of COVID-19 testing is only possible because of FDA’s technical expertise and reduction of regulatory barriers, coupled with the private sector’s ability to innovate and their high motivation to answer complex challenges posed by this pandemic.”
“Providing this type of flexibility for processing saliva samples to test for COVID-19 infection is groundbreaking in terms of efficiency and avoiding shortages of crucial test components like reagents,” said FDA Commissioner Stephen M. Hahn, M.D. “Today’s authorization is another example of the FDA working with test developers to bring the most innovative technology to market in an effort to ensure access to testing for all people in America. The FDA encourages test developers to work with the agency to create innovative, effective products to help address the COVID-19 pandemic and to increase capacity and efficiency in testing.”
SalivaDirect does not require any special type of swab or collection device; a saliva sample can be collected in any sterile container. This test is also unique because it does not require a separate nucleic acid extraction step. This is significant because the extraction kits used for this step in other tests have been prone to shortages in the past. Being able to perform a test without these kits enhances the capacity for increased testing, while reducing the strain on available resources. Additionally, the SalivaDirect methodology has been validated and authorized for use with different combinations of commonly used reagents and instruments, meaning the test could be used broadly in most high-complexity labs.
Yale intends to provide the SalivaDirect protocol to interested laboratories as an “open source” protocol, meaning that designated laboratories could follow the protocol to obtain the required components and perform the test in their lab according to Yale’s instructions for use. Because this test does not rely on any proprietary equipment from Yale and can use a variety of commercially available testing components, it can be assembled and used in high-complexity labs throughout the country, provided they comply with the conditions of authorization in the EUA.
This is the fifth test that the FDA has authorized that uses saliva as a sample for testing. Testing saliva eliminates the need for nasopharyngeal swabs, which have also been prone to shortages, and alleviates the patient discomfort associated with these swabs. Since the saliva sample is self-collected under the observation of a healthcare professional, it could also potentially lower the risk posed to healthcare workers responsible for sample collection. While FDA has seen variable performance in tests using saliva, Yale School of Public Health submitted data with its EUA request from which the FDA determined that Yale’s test meets the criteria for emergency authorization when used to test saliva samples for SARS-CoV-2, the virus that causes COVID-19 infection.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
The flu vaccine has been 48% effective so far this season, according to a report from the Centers for Disease Control and Prevention. Brendan Flannery, an epidemiologist at the CDC’s influenza division, said, “The 48 percent overall is not as good as we would like to see for flu vaccine, but the protection we see is significant.”
This is according to a report released by the U.S. Centers for Disease Control and Prevention.
The CDC also reported that eight pediatric deaths have been reported due to seasonal influenza and that 10 states have experienced high amounts of people with flu-like symptoms. Widespread influenza activity was reported in 37 states.